Drug product assay
The label claim for drug product ensures each vial meets a minimum concentration specification on the label. Label claim can be determined as follows:
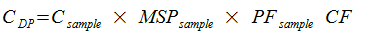
where
|
CDP |
Concentration of UV-pure material in Drug Product (mg/ml) |
|
Csample |
Concentration of UV-pure material in sample (mg/mL) |
|
MSPsample |
Purity of FLP as determined in section (X) by UV purity |
|
PFsample |
Preparation factor for sample (see below) |
|
CF |
Conversion Factor - ratio of sodiated to free acid form of the molecule |
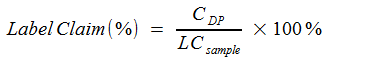
where
|
CDP |
Concentration of UV-pure material in Drug Product (mg/ml) |
|
LCsample |
Label claim (mg/mL) |
Preparation Factor
The preparation factor for the sample is determined by whether the sample volume is determined gravimetrically (that is, sample volume is weighed on analytical balance) or volumetrically (that is, sample is measured using pipette or similar dispenser).
The gravimetric factor is determined by:

where
|
PFsample |
Preparative factor – gravimetric |
|
Vsample |
Volume of sample (mL) |
|
Wtsample |
Weight (g) of sample |
|
Dsample |
Density (g/mL) of sample reported to two decimal places |
The volumetric factor is determined by:
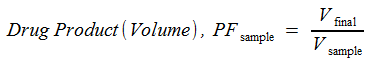
where
|
PFsample |
Preparative factor – volumetric |
|
Vfinal |
Final volume of sample (mL) |
|
Vsample |
Volume of sample (mL) |
base-id: 11124051723
id: 11124051723