Drug substance assay
Drug substance or active pharmaceutical ingredient calculation will give the anhydrous FLP in each sample. This may act as an additional confirmation of the sample purity, but considering sample concentration as it is prepared by the analyst. As such, FLP purity and drug substance assay should correspond, that is, subtracting purity and assay should be within a certain allowable percentage.
First, the concentration of UV-pure material in a sample is calculated by:
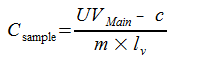
where
|
UVMain |
Area of UV main peak (mAU*s) |
|
c |
y-intercept of least squares line (calibration curve) |
|
m |
slope of least squares line (calibration curve) |
|
lv |
sample injection volume (µL) |
Concentration (%)(w/w) is then calculated by:
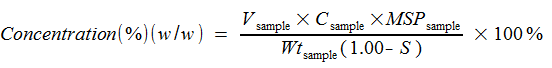
where
|
Concentration (%)(w/w) |
Concentration weight per weight |
|
Vsample |
Total volume of sample solution (mL) |
|
Csample |
Concentration of UV-pure material in sample (mg/mL) |
|
MSPsample |
MS Purity, expressed as decimal; determined by dividing MS Purity by UV Purity |
|
Wtsample |
Weight of sample in mg |
|
S |
Decimal fraction of sum of water, solvents, and sodium acetate, as applicable |
base-id: 11124046603
id: 11124046603