Set up OAA project parameters
OAA project parameters are valid for all result sets in a project. Some of them can later be adjusted for individual result sets; the project parameters then serve as default values.
Preparations
|
-
In the Oligo Analysis Accelerator (OAA) web interface on the Data Selection tab, select the relevant project.
The Projects list includes all projects in your OpenLab CDS system that you can access. Ensure that you edit a project that is intended to be run with the Oligo Analysis Accelerator.
Do not define oligo analysis parameters for other projects. You will not be able to remove the parameters from the project.
-
To edit the project parameters, click the three dots next to the project name, and select Project Settings.
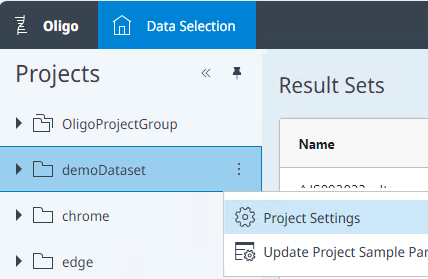
-
Provide the parameters for your project. For details on each parameter, see the following overview.
Compound & Impurities
File With Ion List Data | Provide a default ion list for all result sets in this project. A preview table shows the content of loaded file. For details, see Ion List. The ion list can be overwritten for individual result sets.
|
Compound | The drop-down list shows all names of type Ion from the ion list. Choose the full-length compound (FLP). By default, the first entry of type Ion is selected. The compound can be overwritten for individual result sets. |
MW Conversion Factor | Ratio of molecular weights of sodiated and free acid forms. See certificate of analysis for the reference standard. |
Latest Eluting Impurity | Ion that will be used as latest eluting impurity for the calculations in the System Suitability step. By default, it is detected automatically. If necessary, choose a specific ion. The drop-down list shows all names of type Ion from the ion list. |
System Suitability Criteria
Limits for "Passed" system suitability. Values outside the given range will result in a "Failed" flag.
Na Adduct Limit (%) ≤ | Upper limit MS peak height of the sodium adduct compared to the MS peak height of the full-length product (FLP) |
% RSD (UV Purity) ≤ | % RSD of UV main peak area for QC standards For example, 1.0 |
UV Purity (%) ± | Tolerated deviation of the average FLP UV purity of all QC standards from the value defined under Reference standard. For example, 0.1 |
Mass Accuracy (m/z) ± | Max. acceptable difference between FLP expected m/z and the m/z value of the highest peak for QC standards average spectrum For example, 0.2 |
UV Calibration Curve R² ≥ | Determination coefficient from UV calibration curve calculation, acceptability limits for passing result For example, 0.99 Based on mAU response- linear regression The R² calculation is the same as in OpenLab CDS, see Calibration curve statistics |
MS Calibration Curve R² ≥ | Determination coefficient for MS calibration curve, based on sum of MS response for FLP and FLP, P=O; second order polynomial regression For example, 0.99 The R² calculation is the same as in OpenLab CDS with Origin=Force, see Calibration curve statistics |
RT Range Start (min) | Lower range border for retention of UV main peak For example, 12.0 |
RT Range End (min) | Upper range border for retention of UV main peak. For example, 22.0 |
UV Curve y-intercept Low Limit (mAU*s) | Lower limit for the response at which the UV calibration curve intersects the y-axis of the coordinate system. |
UV Curve y-intercept High Limit (mAU*s) | Upper limit for the response at which the UV calibration curve intersects the y-axis of the coordinate system. |
Target Concentration Low Limit (%) | Lower limit for the target concentration For example, 75 |
Target Concentration High Limit (%) | Upper limit for the target concentration For example, 130 |
Target Concentration (mg/mL) | Target concentration in mg/mL The default value is 0.1 |
Ion Classification
Pre-FLP Response Threshold (%) | Peaks eluting earlier than the FLP are considered only if they are higher than the defined threshold. Lower peaks are considered not detected. |
Post-FLP Response Threshold (%) | Peaks eluting later than the FLP are considered only if they are higher than the defined threshold. Lower peaks are considered not detected. |
Reference standard
Reference Standard | Name of the working standard solution (WSS); from Certification of Analysis (CofA) |
Reference Standard Lot Number | Lot number of reference standard, per CofA |
Conc. Of Reference Standard (mg/ml) | Concentration (mg/mL) of the reference standard used for test, from CofA or Notebook |
Reference UV Purity (%) | Purity of reference standard, typically supplied per CofA. This is not a calculated value. It is required for the calculation of system suitability criteria. |
Acceptance Criteria
Acceptance criteria must be defined for different formulations.
Formulation | Drug product (DP)- final, formulated drug that requires assay (that is, label claim for amount of active pharmaceutical ingredient or API in the drug product) Drug substance (DS) - The API, where the equilibrated material must be weighed out and water content determination is necessary. |
UV purity (%) | Limit for passing result |
MS purity (%) | Limit for passing result |
TDP Limit (%) | Total degradation product limit |
Identity range (Da) ± | Range in Dalton added to the mass of the FLP |
Label claim relative difference (%) | Limit for passing result, only editable for Drug Product |
See Also
base-id: 11123958923
id: 36028808142922891